ʻO ke kūlana o kēia manawa: ʻo ka ʻoihana lāʻau lapaʻau e pili nui ana i ka chemical synthesis pharmaceutical, biological pharmaceutical a me nā lāʻau lapaʻau kuʻuna Kina, a ʻo ka hana ke ʻano o nā huahana like ʻole, nā kaʻina hana paʻakikī a me nā unahi hana like ʻole.
ʻO ka wai ʻino i hana ʻia e ke kaʻina hana lāʻau i loaʻa nā hiʻohiʻona o ka pollutant kiʻekiʻe, nā mea paʻakikī, ka biodegradability maikaʻi ʻole a me ke kiʻekiʻe o nā mea ola kino.
ʻO ka synthesis kemika a me ka fermentation pharmaceutical production wastewater ka paʻakikī a me ka mea nui i ka hoʻokele pollution ʻoihana lāʻau.
ʻO ka wai hoʻoheheʻe kemika he mea haumia nui i hoʻokuʻu ʻia i ka wā o ka hana lāʻau lapaʻau [2].
Hiki ke hoʻokaʻawale ʻia ka wai ʻōpala lāʻau lapaʻau i ʻehā mau ʻāpana [3], ʻo ia hoʻi ka wai ʻōpala a me ka wai makuahine i ke kaʻina hana;
ʻO ke koena wai i ka hoʻihoʻi ʻana me ka solvent, ka wai koi mua, ka huahana, etc.
ʻO ke kaʻina hana kōkua e like me ka wai anuanu, etc.
ʻO nā mea hana a me ka lepo e hoʻoheheʻe ʻia i ka wai ʻino;
ʻO ka wai hoʻoheheʻe hale.
ʻenehana no ka mālama ʻana i ka wai ʻōpala waena
I ka nānā ʻana i nā hiʻohiʻona o ka wai ʻawaʻawa waena o ka lāʻau lapaʻau e like me ka COD kiʻekiʻe, ka nitrogen kiʻekiʻe, ka phosphorus kiʻekiʻe, ka paʻakai kiʻekiʻe, ka chroma hohonu, ka hoʻohui paʻakikī a me ka biodegradability maikaʻi ʻole, ʻo nā ʻano lapaʻau maʻamau i hoʻohana ʻia me ka mālama physicochemical a me ke kaʻina hana biochemical [6].
E like me nā ʻano like ʻole o ka maikaʻi o ka wai ʻino, e hoʻohana ʻia kekahi ʻano hana e like me ka hui ʻana o ke kaʻina physicochemical a me ke kaʻina biological [7].
ʻO ke kiʻi
1. ʻenehana lapaʻau kino a me ke kemika
I kēia manawa, ʻo ke ʻano o ka mālama kino kino a me ke kemika no ka wai ʻōpala hana lāʻau lapaʻau e pili ana: ke ʻano flotation gas, coagulation sedimentation method, adsorption method, reverse osmosis method, incineration method and advanced oxidation process [8].
Eia kekahi, hoʻohana pinepine ʻia nā ʻano hana electrolysis a me nā kemika, e like me FE-C micro-electrolysis a me MAP no ka hoʻohemo ʻana i ka nitrogen a me ka phosphorus, i ka mālama ʻana i ka wai ʻawaʻawa waena.
1.1 ʻO ke ʻano coagulation a me ka sedimentation
ʻO ke kaʻina hana coagulation kahi hana e hoʻololi ʻia ai nā ʻāpana i hoʻokuʻu ʻia a me nā ʻāpana colloidal i loko o ka wai i ke kūlana paʻa ʻole ma o ka hoʻohui ʻana i nā mea kemika a laila hoʻohui ʻia i loko o nā flocs a i ʻole nā flocs i maʻalahi e hoʻokaʻawale.
I kēia manawa, hoʻohana pinepine ʻia kēia ʻenehana i ka pretreatment, intermediate treatment and advanced treatment of pharmaceutical wastewater [10].
ʻO ka ʻenehana o ka coagulation a me ka sedimentation nā mea maikaʻi o ka ʻenehana makua, nā mea maʻalahi, ka hana paʻa a me ka mālama pono.
Eia nō naʻe, e nui ka nui o ka lepo kemika i hana ʻia ma ke kaʻina o ka noi ʻana i kēia ʻenehana, e alakaʻi i ka pH haʻahaʻa o ka effluent a me ka nui o ka paʻakai o ka wai ʻino.
Eia kekahi, ʻaʻole hiki i ka ʻenehana coagulation a me ka sedimentation ke hoʻopau pono i nā mea haumia i hoʻoheheʻe ʻia i ka wai ʻino, ʻaʻole hiki iā ia ke hoʻopau loa i nā mea ʻawaʻawa a me nā mea ʻino i loko o ka wai ʻino.
1.2 Ke ʻano hoʻoheheʻe kemika
ʻO ke ʻano hoʻoheheʻe kemika he ʻano kemika e wehe i nā mea haumia i loko o ka wai ʻino ma o ka hopena kemika ma waena o nā mea hoʻoheheʻe kemika a me nā mea haumia i loko o ka wai ʻōpala e hana i nā paʻakai hiki ʻole, hydroxides a i ʻole nā hui paʻakikī.
Loaʻa i ka wai ʻōpala waena lāʻau lapaʻau ke kiʻekiʻe o ka ammonia nitrogen, phosphate a me nā ion sulfate, a me nā mea ʻē aʻe.
Ma ke ʻano he ʻenehana lapaʻau wai kuʻuna, hoʻohana pinepine ʻia ka ua kemika e hoʻomaʻemaʻe i ka wai ʻino.
Ma muli o ka hoʻohana ʻana i nā mea kemika maʻemaʻe kiʻekiʻe i ke kaʻina hana o ka wai ʻawaʻawa waena, ʻo ka wai ʻino ka nui o ka nui o ka amonia nitrogen a me ka phosphorus a me nā mea haumia ʻē aʻe, me ka hoʻohana ʻana i ka magnesium ammonium phosphate chemical precipitation method hiki ke hoʻopau pono i nā mea haumia ʻelua i ka manawa like. I ka manawa, hiki ke hana hou ʻia ka ua paʻakai magnesium ammonium phosphate.
ʻIke ʻia ke ʻano hoʻoheheʻe kemika ʻo Magnesium ammonium phosphate ma ke ʻano he struvite.
I ke kaʻina hana o ka pharmaceutical intermediate, hoʻohana pinepine ʻia ka nui o ka sulfuric acid i kekahi mau papa hana, a haʻahaʻa paha ka pH o kēia ʻāpana o ka wai. I mea e hoʻomaikaʻi ai i ka waiwai pH o ka wai ʻino a hoʻoneʻe i kekahi mau ion sulfate i ka manawa like, hoʻohana pinepine ʻia ke ʻano o ka hoʻohui ʻana iā CaO, i kapa ʻia ʻo ke ʻano hoʻoheheʻe kemika o ka desulfurization wikiwiki.
1.3 adsorption
ʻO ke kumu o ka wehe ʻana i nā mea haumia i loko o ka wai ʻōpala ma ke ʻano adsorption e pili ana i ka hoʻohana ʻana i nā mea paʻa paʻa porous e adsorb i kekahi a i ʻole nā ʻano mea haumia i loko o ka wai ʻōpala, i hiki ke hoʻoneʻe ʻia a hana hou ʻia nā mea haumia i loko o ka wai.
Hoʻohana pinepine ʻia nā adsorbents e like me ka lehu lele, slag, carbon activated a me adsorption resin, a ʻo ia ka mea maʻamau ka hoʻohana ʻana i ke kalapona hoʻāla.
1.4 lele lele
ʻO ke ʻano hoʻoheheʻe ea he kaʻina mālama wai ʻino i hoʻohana ʻia nā ʻōhū liʻiliʻi i hoʻopuehu nui ʻia ma ke ʻano he mea lawe e hoʻopili ai i nā mea haumia i ka wai ʻino. No ka mea, ʻoi aku ka liʻiliʻi o nā ʻōpū liʻiliʻi e pili ana i nā mea haumia ma mua o ka wai a lana i luna, ʻike ʻia ka hoʻokaʻawale ʻana o ka wai paʻa a i ʻole ka wai-wai.
Ea floatation palapala i hoʻoheheʻe ea floatation, aerated ea floatation, electrolysis ea floatation a me kemika ea floatation, etc. [18], i waena o ia kemika ea floatation kūpono no ka mālama 'ana i ka wastewater me kiʻekiʻe suspended mea maʻiʻo.
Loaʻa ka maikaʻi o ke ʻano o ka lele ʻana i ka haʻahaʻa haʻahaʻa, ka hana maʻalahi, ka mālama pono a me ka hoʻohana haʻahaʻa haʻahaʻa, akā ʻaʻole hiki ke hoʻopau pono i nā mea haumia i hoʻoheheʻe ʻia i loko o ka wai.
1.5 electrolysis
ʻO ke kaʻina hana electrolytic ka hoʻohana ʻana i ka hana i manaʻo ʻia i kēia manawa, hana i nā ʻano hopena kemika, hoʻololi i nā mea haumia ʻino i ka wai ʻino a ua wehe ʻia, ʻo ke kumu hoʻohālikelike o ke kaʻina hana electrolytic i hana ʻia i ka hopena electrolyte ma o ka mea electrode a me ka hopena electrode, e hana hou i ka ékological hou. ʻO ka oxygen ecological a me ka hydrogen [H] a me nā mea hoʻohaumia wai o ka REDOX e hoʻoneʻe i ka pollutant.
ʻO ke ʻano electrolysis ka hana kiʻekiʻe a me ka hana maʻalahi i ka mālama ʻana i ka wai. I ka manawa like, hiki i ke ʻano electrolysis ke wehe pono i nā mea kala i loko o ka wai ʻino a hoʻomaikaʻi maikaʻi i ka biodegradability o ka wai ʻino.
ʻO ke kiʻi
2. ʻenehana oxidation kiʻekiʻe
ʻO ka ʻenehana oxidation kiʻekiʻe, ma ke ʻano he ʻenehana mālama wai hou, he nui nā pono, e like me ke kiʻekiʻe o ka maikaʻi o ka hoʻohaumia ʻana o nā mea haumia, ʻoi aku ka maikaʻi o ka degradation a me ka oxidation o nā mea haumia a ʻaʻohe pollution lua.
ʻO ka ʻenehana oxidation kiʻekiʻe, ʻike ʻia hoʻi ʻo ka ʻenehana hoʻonanea hohonu, he ʻenehana lapaʻau kino a me kemika e hoʻohana ana i ka oxidizer, māmā, uila, kani, magnetic a me ka catalyst e hoʻoulu ai i nā radical manuahi ikaika loa (e like me ·OH) e hoʻohaʻahaʻa i nā mea haumia kūlohelohe refractory.
Ma ke kahua o ka lāʻau lapaʻau wai ʻino, ua lilo ka ʻenehana oxidation kiʻekiʻe i mea nui o ka noiʻi nui a me ka nānā.
Loaʻa ka ʻenehana hoʻonaʻauao kiʻekiʻe i ka electrochemical oxidation, oxidation kemika, ultrasonic oxidation, wet catalytic oxidation, photocatalytic oxidation, composite catalytic oxidation, supercritical water oxidation a me ka ʻenehana hui pū ʻia.
ʻO ke ʻano o ka oxidation kemika ka hoʻohana ʻana i nā mea kemika iā lākou iho a i ʻole ma lalo o kekahi mau kūlana me ka oxidation ikaika e hoʻoneʻe i nā mea haumia organik i loko o ka wai ʻino e hoʻokō ai i ke kumu o ka wehe ʻana i nā mea haumia, nā ʻano hoʻoheheʻe kemika me ka ozone oxidation, Fenton oxidation method and wet catalytic oxidation method.
2.1 Ke kaʻina hana hoʻokahe Fenton
ʻO ke ʻano o ka Fenton oxidation ke ʻano o ke ʻano o ke ʻano o ka ʻoki ʻana i hoʻohana nui ʻia i kēia manawa. Ke hoʻohana nei kēia ʻano i ka paʻakai ferric (Fe2+ a i ʻole Fe3+) ma ke ʻano he catalyst e hana · OH me ka oxidation ikaika ma lalo o ke kūlana o ka hoʻohui ʻana i ka H2O2, hiki ke loaʻa i ka hopena oxidation me nā mea haumia organik me ka koho ʻole e hoʻokō i ka degradation a me ka mineralization o nā mea haumia.
He nui nā pōmaikaʻi o kēia ʻano, ʻo ia hoʻi ka wikiwiki wikiwiki, ʻaʻohe pollution lua a me ka oxidation ikaika, a pēlā aku. ʻona o ka wai ʻino a me nā mea ʻē aʻe.
2.2 Electrochemical oxidation ala
ʻO ke ʻano o ka electrochemical oxidation ʻo ia ka hoʻohana ʻana i nā mea electrode e hana i ka radical manuahi superoxide ·O2 a me ka radical free hydroxyl ·OH, ʻelua o ia mau mea he kiʻekiʻe oxidation hana, hiki ke oxidize i ka mea ola i loko o ka wai ʻino, a laila hoʻokō i ke kumu o ka wehe ʻana i nā mea haumia.
Eia naʻe, loaʻa i kēia ʻano nā hiʻohiʻona o ka hoʻohana ʻana i ka ikehu kiʻekiʻe a me ke kumukūʻai kiʻekiʻe.
2.3 Photocatalytic oxidation
ʻO ka Photocatalytic oxidation kahi ʻenehana lapaʻau maikaʻi loa i ka ʻenehana mālama wai, e hoʻohana ana i nā mea catalytic (e like me TiO2, SrO2, WO3, SnO2, a me nā mea ʻē aʻe) e like me nā mea lawe catalytic e lawe i ka catalytic oxidation o ka hapa nui o nā mea hoʻohaumia i loko o ka wai ʻino. e hoʻokō i ke kumu o ka wehe ʻana i nā mea haumia.
No ka mea, ʻo ka hapa nui o nā pūhui i loko o ka wai ʻōpala lāʻau lapaʻau he mau mea polar me nā hui acidic a i ʻole nā mea polar me nā hui alkaline, hiki ke hoʻohaʻahaʻa pololei ʻia ia mau mea e ka mālamalama.
2.4 ʻO ka hoʻoheheʻe wai nui
ʻO Supercritical water oxidation (SCWO) kahi ʻano ʻenehana mālama wai e lawe i ka wai ma ke ʻano he kumu a hoʻohana i nā hiʻohiʻona kūikawā o ka wai i ke kūlana supercritical e hoʻomaikaʻi ai i ka wikiwiki o ka hopena a ʻike i ka hoʻohemo piha ʻana o nā mea organik.
2.5 ʻenehana hui pū ʻana o ka oxidation kiʻekiʻe
Ke hoʻohana nei kēlā me kēia ʻenehana oxidation holomua i nā palena ponoʻī, i mea e hoʻomaikaʻi ai i ka maikaʻi o ka mālama ʻana i ka wai ʻino, ua hui pū ʻia kahi pūʻulu o nā ʻenehana oxidation kiʻekiʻe, i hoʻokumu ʻia i ka hui pū ʻana o nā ʻenehana oxidation kiʻekiʻe, a i ʻole kahi ʻenehana hoʻonaʻauao holomua hoʻokahi i hui pū ʻia me nā ʻenehana ʻē aʻe i mea hou. ʻenehana no ka hoʻomaikaʻi ʻana i ka hiki o ka oxidation a me ka hopena lapaʻau a me ka hoʻokō ʻana i nā loli maikaʻi o ka wai i ka mālama ʻana i ka wai lāʻau lapaʻau papa nui.
UV-Fenton, UV-H2O2, UV-O3, ultrasonic photocatalysis, activated carbon photocatalysis, microwave photocatalysis and photocatalysis, etc.
Ozone activated carbon process, O3-H2O2 and UV-O3, from the treatment effect of refractory wastewater and engineering application, O3-H2O2 and UV-O3 i oi aku ka hiki ke ulu.
ʻO ke kaʻina hana hoʻohui Fenton maʻamau e pili ana i ka micro-electrolysis Fenton method, iron filings H2O2 method, photochemical Fenton method (e like me ka solar Fenton method, UV-Fenton method, etc.), akā hoʻohana nui ʻia ke ala Fenton uila.
ʻO ke kiʻi
3. ʻenehana lapaʻau biochemical
ʻO ka ʻenehana lapaʻau biochemical ka ʻenehana nui i ka mālama ʻana i ka wai, ma o ka ulu ʻana o ka microbial, metabolism, reproduction a me nā kaʻina hana ʻē aʻe e hoʻoheheʻe i ka mea organik i loko o ka wai ʻino, loaʻa i kā lākou ikehu ponoʻī a hoʻokō i ke kumu o ka wehe ʻana i nā mea organik.
3.1 ʻenehana lapaʻau anaerobic biological
ʻO ka ʻenehana lapaʻau anaerobic biological i ka nele o ka oxygen molecular environment, ka hoʻohana ʻana i ka metabolism anaerobic bacteria, ma o ke kaʻina hana o ka hydrolytic acidification, ka hana hydrogen acetic acid a me ka hana methane a me nā kaʻina hana e hoʻohuli ai i nā macromolecules, paʻakikī e hoʻohaʻahaʻa i ka mea kūlohelohe i CH4, CO2 , H2O a me nā mea olaola mole liʻiliʻi.
Loaʻa pinepine ka wai ʻōpala lāʻau synthetic i ka nui o nā mea kūlohelohe cyclic refractory, ʻaʻole hiki ke hoʻohaʻahaʻa ʻia a hoʻohana ʻia e nā bacteria aerobic, no laila ua lilo ka ʻenehana lapaʻau anaerobic i kēia manawa i kumu nui i ke kula o ka mālama ʻana i ka wai lāʻau lapaʻau ma ka home a ma waho [43] .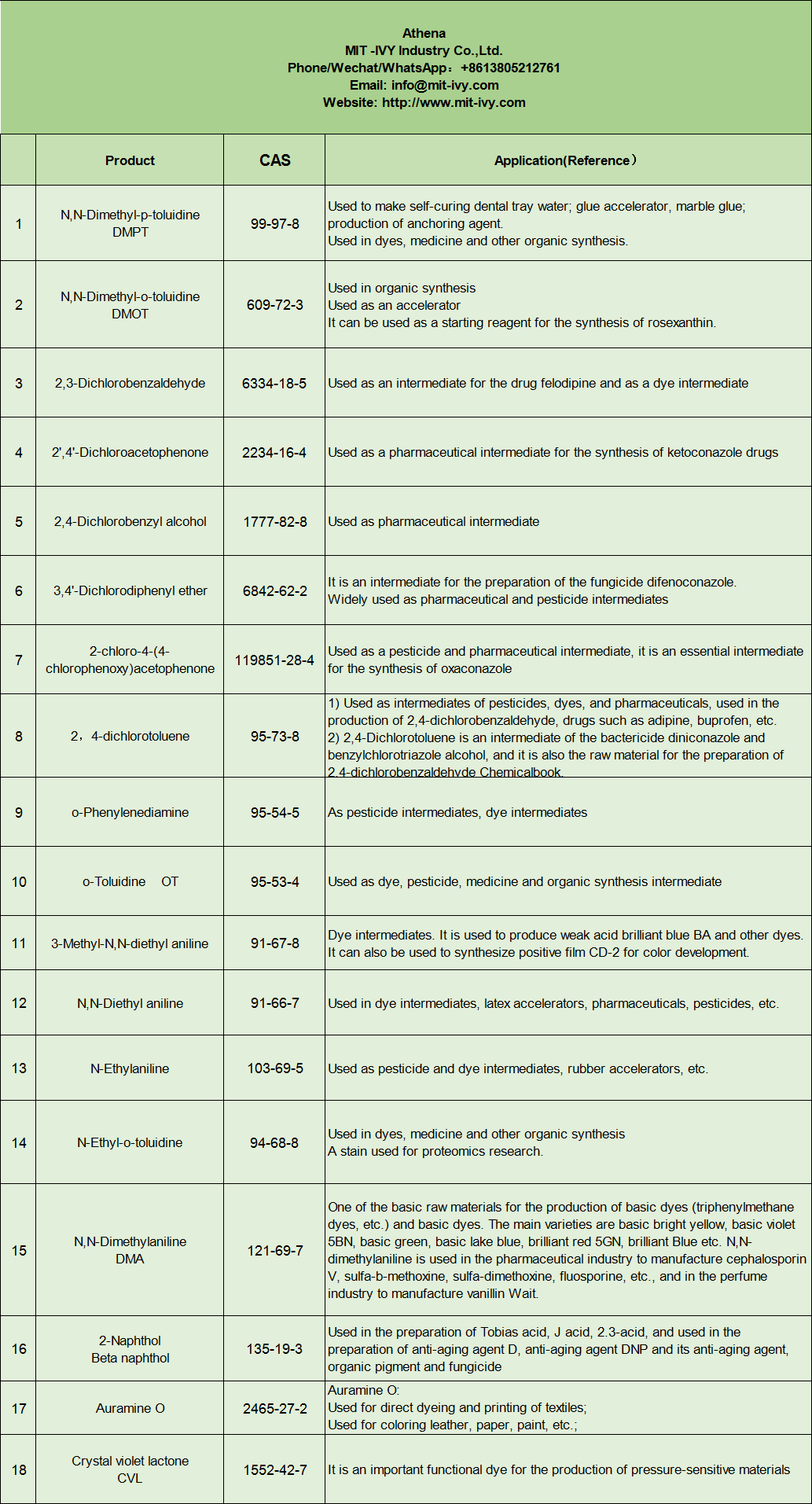
He nui nā pono o ka ʻenehana lapaʻau anaerobic: ʻaʻole pono ke kaʻina hana anaerobic reactor e hāʻawi i ka aeration, haʻahaʻa ka hoʻohana ʻana i ka ikehu;
He kiʻekiʻe loa ka haʻawe organika o ka wai influent anaerobic.
ʻO nā pono meaʻai haʻahaʻa;
He haʻahaʻa ka hoʻoulu ʻana o ka ʻōpala anaerobic, a he maʻalahi ka hoʻomaloʻo ʻana i ka lepo.
Hiki ke hana hou ʻia ka methane i hana ʻia ma ke kaʻina hana anaerobic e like me ka ikehu.
Eia nō naʻe, ʻaʻole hiki ke hoʻokuʻu ʻia ka effluent anaerobic i ka maʻamau, a pono e mālama hou ʻia ma ka hui ʻana me nā kaʻina hana ʻē aʻe. Eia nō naʻe, pili ka ʻenehana lapaʻau anaerobic i ka waiwai pH, ka mahana a me nā mea ʻē aʻe. Inā nui ka fluctuation, pili pono ka hopena anaerobic, a laila e hoʻopilikia ʻia ka maikaʻi o ka effluent.
3.2 Aerobic lāʻau lapaʻau ʻenehana
ʻO ka ʻenehana lapaʻau biological Aerobic kahi ʻenehana lapaʻau biological e hoʻohana ana i ka decomposition oxidative a me ka assimilation synthesis o nā bacteria aerobic e wehe i nā mea kūlohelohe. I ka wā o ka ulu ʻana a me ka metabolism o nā mea ola aerobic, e lawe ʻia ka nui o ka hana hou ʻana, kahi e hoʻoulu ai i ka sludge hou. E hoʻokuʻu ʻia ka sludge i hoʻāla ʻia ma ke ʻano o ke koena sludge, a e hoʻomaʻemaʻe ʻia ka wai ʻino i ka manawa like.
| Huahana | CAS |
| N,N-Dimethyl-p-toluidine DMPT | 99-97-8 |
| N,N-Dimethyl-o-toluidine DMOT | 609-72-3 |
| 2,3-Dichlorobenzaldehyde | 6334-18-5 |
| 2′,4′-Dichloroacetophenone | 2234-16-4 |
| 2,4-Dichlorobenzyl waiʻona | 1777-82-8 |
| 3,4′-Dichlorodiphenyl etera | 6842-62-2 |
| 2-chloro-4-(4-chlorophenoxy)acetophenone | 119851-28-4 |
| 2,4-dichlorotoluene | 95-73-8 |
| o-Phenylenediamine | 95-54-5 |
| o-Toluidine OT | 95-53-4 |
| 3-Methyl-N,N-diethyl aniline | 91-67-8 |
| N,N-Diethyl aniline | 91-66-7 |
| N-Ethylaniline | 103-69-5 |
| N-Ethyl-o-toluidine | 94-68-8 |
| N,N-Dimethylaniline DMA | 121-69-7 |
| 2-Naphthol Beta naphthol | 135-19-3 |
| Auramine O | 2465-27-2 |
| lactone ʻulaʻula violet CVL | 1552-42-7 |
MIT –IVY ʻOihana Kemika me4 hale hanano 19 makahiki, nā mea waihoʻoluʻuKūwaenas & lāʻau waena &kemika maikaʻi & kūikawā .TEL(WhatsApp):008613805212761 Athena
Ka manawa hoʻouna: Apr-25-2021





